|
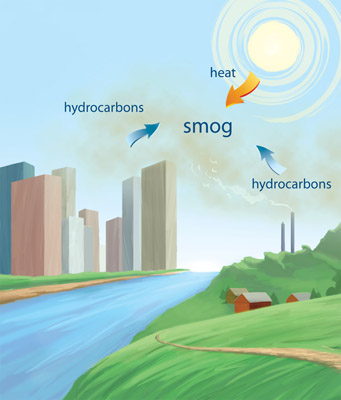
How it hurts youWhen ozone accumulates, it hurts people, pets, and crops. Normally, ozone converts back to oxygen. However, airborne hydrocarbons (from motor vehicles, industrial emissions, and other kinds of incomplete combustion and solvent use) can disrupt this conversion, allowing the ozone to accumulate. Because sunlight and heat play roles in the creation of smog, ozone concentrations most often rise in the warmer months of the year and peak during the warmer hours of the day. While winds might dissipate the ozone, frequent temperature inversions during warm weather can hold down and stagnate the lower atmosphere, allowing ozone to build up. Sometimes inversions occur. Normally, warm air rises until it cools at higher altitudes, thus moving and mixing the air. A temperature inversion occurs when the upper air is warmer and prevents the lower air from rising, trapping it in place. This frequently happens during the winter months in cities that are situated in mountain valleys. This happens in many cities, which is where the term “urban smog” comes from. The buildup of ozone causes the grey, thick, photochemical smog that is often present in large, metropolitan areas.
Thus, the centers of ozone pollution are the great centers and suburbs of human activity: Los Angeles, Houston, Atlanta, New York, Boston, and other large metropolitan areas. (This is why in some cities in developing countries, people are somewhat proud of smog—it is a sign of industrialization's progress.) In rural areas, less ozone is likely to accumulate. There are fewer automobiles, homes, and factories. In addition, moisture in rural soil and trees absorbs some of the radiant energy from the sun, keeping these areas cooler than nearby cities. But rural areas do not escape the effects of ozone entirely. Trees themselves generate some hydrocarbons and add to ozone levels. Many power plants that burn fossil fuels are in rural areas, which increases the concentrations of oxides of nitrogen in rural air. According to the weatherman, or the National Oceanic and Atmospheric Administration, rural sections of the East and South of the US have among the highest summer ozone readings in North America, partly as a result of spillover from cities and suburbs. Ozone in the lower layers of the atmosphere can be harmful, but we need ozone in the layer of the atmosphere called the stratosphere. Ozone naturally exists in high quantities in that layer where it acts as a shield and protects the earth from the harmful rays of the sun. Unfortunately, while we are seeing too much ozone in the lower layers of the atmosphere, there has been a drop of ozone levels in the stratosphere! Even small changes in the ozone layer in the stratosphere have been linked to an increase in skin cancer. Further deterioration could be even more harmful. Indeed, without the ozone layer's filter, life on earth as we know it could not exist. In the 1980s, an ozone hole was found to be forming every spring over Antarctica, where cold, stratospheric temperatures promote the chemical reactions that destroy ozone. Scientists are watching for UV damage to penguins, whales, and the vital food chain of fish and other sea creatures, including the microscopic algae (phytoplankton) that are the base food for the undersea food chain. The Arctic has no landmass and is warmer than Antarctica, but an ozone hole now appears over the North Pole as well. Scientists have discovered that the ozone layer is created naturally by the action of sunlight on oxygen, and can be destroyed by compounds called chlorofluorocarbons (CFCs). Chlorofluorocarbon gases which come from air conditioners and spray cans could waft intact to the stratosphere, where they might persist for as long as one hundred years, gradually being broken down by the sun's UV rays into free chlorine atoms. Since 1985, when the hole in the ozone layer was reported, major steps have been made toward protecting the ozone shield from further damage by eliminating use of these ozone-depleting chemicals. Phasing out the use of ozone-depleting chemicals has been no quick fix. It may slow the destruction of the ozone layer and eventually halt it, perhaps allowing it to rebuild. But a NASA satellite showed the hole in the shield over Antarctica reached 10.5 million square miles on September 19, 1998—bigger than ever. No one can predict the future with certainty, but over the twenty-first century, the shield may slowly recover.1 Footnotes 
|
About Us | Terms of Use | Contact Us | Partner with Us | Press Release | Sitemap | Disclaimer | Privacy Policy
©1999-2011 OpenLearningWorld . com - All Rights Reserved